Introduction
With the introduction of tyrosine kinase inhibitors (TKI), the outcomes of patients with chronic myeloid leukemia (CML) has improved dramatically, with the majority of patients in chronic phase achieving near normal life expectancy. As pts live longer on TKIs, they may be more susceptible to subsequent malignancies either as late effects of TKI treatment, or due to genomic instability from having an established hematologic malignancy. Theoretical mechanisms of TKI carcinogenesis include impaired DNA repair mechanisms, inhibition of T-lymphocytes or impaired differentiation of peripheral blood progenitor cells. In this study, we evaluated the risk of subsequent cancers in CML pts at the population level and compared the effect of treatment period (pre- and post-TKI era) on that risk.
Methods
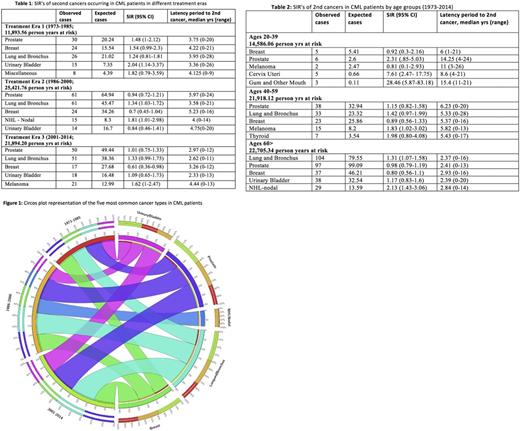
We used the April 2017 release of all 18 registries of the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute to assemble the study cohort. Only patients with CML, not otherwise specified or BCR/ ABL-positive CML were included in the analysis. Patients aged >20 years who had CML as a first cancer diagnosed between January 1973 and December 2013 who developed second cancers (excluding myeloid neoplasms and acute lymphocytic leukemia [to avoid misclassification with CML in blast crisis] and non-melanoma skin cancer) were studied. CML pts with third and subsequent cancers were excluded. Pt and disease characteristics including sex, race and ethnicity, age at diagnosis, year of diagnosis, and year of last follow-up or death were collected. Standardized incidence ratios (SIR) were calculated to assess the risk of a second malignancy by dividing the number of observed second malignancies in CML pts with the number of expected malignancies in the US population, using SEER*Stat. SIRs for the five most common second cancer types were analyzed by treatment eras (1973-1985 [era 1, in which busulfan and hydroxyurea predominated], 1986-2000 [era 2, in which interferon and hematopoietic cell transplantation predominated] and 2001-2014 [era 3, the TKI era] per Blood. 2011; 117(3):755-63 and by age groups (20-39 yrs; 40 -60 yrs and > 60 yrs).
Results
Of 26,810 patients diagnosed with CML between 1973 and 2014, 1,324 (4.94%) developed a subsequent cancer. The prevalence of second malignancies was 4.59% in era 1, 5.35% in era 2, and 4.84% in era 3 (with the increase from era 1 to 2 likely due to improved capture of cancers and possible transplant effects (BBMT 2007: 13(10):1121-1134)). The most common second cancers in the study period were prostate (17.98%), lung and bronchus (16.01%), breast (8.31%), urinary bladder (5.36%) and non-Hodgkin lymphoma (4.38%) with distribution by treatment era shown in Figure 1. The majority of subsequent malignancies occurred in Caucasians (85.95%), males (61.25%) and in pts > 60 years (68.43%). Table 1 shows SIR of second cancers by treatment eras. In era 1, an excess risk for bladder cancer was observed compared to matched population controls without CML (SIR 2.04, 95% CI 1.14-3.37). In era 2, SIRs for lung cancer and non-Hodgkin lymphoma were marginally elevated [1.34 (95% CI 1.03-1.72) and 1.81 (95% CI 1.01-2.98)], respectively. No excess risk of subsequent cancers was detected in the TKI era. Differences in excess risk of second cancers compared to matched population controls without CML were observed in the study period (1973-2014) when analyzed by age groups, as shown in table 2. Excess risk of oral (SIR 28.46, 95% CI 5.87-83.18) and cervical cancers (SIR 7.61, 95% CI 2.47-17.75) was observed in 20-39 yr age group, melanoma (SIR 1.83, 95% CI 1.02-3.02) in 40-59 yr age cohort, and lung and bronchus (SIR 1.31, 95% CI 1.07-1.58) and non-Hodgkin lymphoma (SIR 2.13, 95% CI 1.43-3.06) in patients >60 yrs.
Conclusions
In the TKI era of treatment for CML, there does not appear to be an excess risk of second cancers compared to what is seen in matched population controls without CML. Types of second cancers differ by age group. The paucity of second cancers may contribute to the survival of CML patients treated with TKIs approaching that of the general population.
Advani: Pfizer: Consultancy; Takeda/ Millenium: Research Funding. Gerds: CTI BioPharma: Consultancy; Incyte: Consultancy. Maciejewski: Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Apellis Pharmaceuticals: Consultancy; Ra Pharma: Consultancy. Majhail: Sanofi: Honoraria; Anthem, Inc.: Consultancy. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal